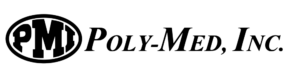On March 2, 1999, the U.S. Food and Drug Administration released a guidance document entitled Guidance for the Preparation of a Premarket Notification Application for a Surgical Mesh. Now in 2018, nearly 20 years later, it is still easy to become overwhelmed with everything required to prepare a new device for submission to the FDA. Guidance documents such as this one provide recommendations for the starting points of testing new devices and help alleviate some of the guesswork around what is required and what isn’t. Fortunately, partnering with a company like Poly-Med, with over 25 years of experience in bioresorbable materials and textiles, can help further navigate these issues.
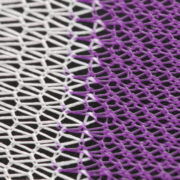
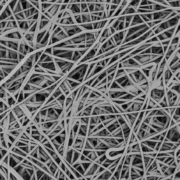
This particular guidance document covers submission guidelines for surgical meshes in a wide variety of applications where a mesh product would be used to reinforce weakened soft tissue. These include area applications in abdominal wall repair (hernia repair), suture line/staple line reinforcement, muscle flap reinforcement, gastric banding, breast reconstruction, pelvic organ prolapse, and many more. For many of these applications, the current market trend is moving toward bioresorbable solutions, providing mechanical support throughout the healing process without permanent synthetic materials in the body. Poly-Med is the leader in bioresorbable textiles and has developed bioresorbable mesh products on the market for a wide variety of applications.
The FDA’s primary interest in any device review can usually be summarized in two words: safety and efficacy. Not surprisingly at all, the guidance document first focuses on the product being safe for human use. Likewise, this is usually a best first-step to consider in product development as future product development will all hinge on the materials as being safe for implantation. With this in this focus, the FDA requires the submission to include description of all material components in the device. This includes at minimum the sources/supplier and purity. To satisfy this requirement, material suppliers can provide reference to device master files (MAF), Certificate of Analysis (CoA), and Safety Data Sheets (SDS) with materials, all of which are noted to help simplify the review process. When considering the materials, special attention should be paid to any materials, reagents, or processing aids which are considered to be potentially cytotoxic, carcinogenic, or immunogenic. Additional testing may be required for any of these materials which are used to air in processing and may remain as residuals in the final material.
The next step in safety of an implantable device is consideration of sterilization. Applications are suggested to specify the method, validations, sterility assurance level (SAL, recommended SAL of 10-6), method for monitoring sterility, and packaging used to maintain sterility. Poly-Med uses several trusted partners with experience in sterilization to coordinate offering these services to our clients. Dependent on the type of material used and the desired processing plan, bioresorbable meshes can be sterilized using irradiation, ethylene oxide, or new emerging technologies such as nitrogen dioxide.1
While many of our materials are used in devices on the market and have proven biocompatibility, it is recommended that biocompatibility testing be conducted on final manufactured, sterilized, and packaged devices, as all can influence the final reaction in the body. While some provisions are allowed for products using the exact same material specifications as another similar device on the market, the guidance generally recommends that applications include testing in accordance to ISO-10993 for Cytotoxicity, Sensitization, Irritation or Intracutaneous Reactivity, Systemic Toxicity (acute), Genotoxicity, Implantation with histology of the surrounding tissue, Hemolysis, and Pyrogenicity. Given that most bioresorbable materials will leave some mass within the body for greater than 30 days, the FDA further recommends testing for Subchronic Toxicity and Chronic Toxicity. These tests should generally be conducted according to relevant USP Class VI and ASTM standards.
When using bioresorbable materials, part of the efficacy claim of a surgical mesh is its ability to degrade over time. In alignment with this guidance, Poly-Med often performs degradation studies for each device developed, being sure to take into account material selection, processing methods, as well as sterilization. Poly-Med is fully capable of conducting in-house in vitro degradation studies in both real and accelerated environments. Accelerated time points are used to quickly identify appropriate time points for a real time study and then evaluate device functionality and specifications at pre-determined time points. For surgical mesh devices, common strength loss tests are dependent on the application, and often include tensile strength, burst strength, suture pull-out strength, and tear resistance.
Though expiration dating is required for all surgical meshes, the need is especially apparent with bioresorbable materials. Many of these materials degrade with exposure to moisture, light, and/or heat, so packaging and sterilization methods can play a critical role in the ultimate shelf-life of the product. This guidance allows for expiration dating to be continuously updated over time without the need for a new 510k submission under the scope of Good Manufacturing Practice (GMP). This allows products to quickly enter the market and then slowly increase the expiration dates of new production as the real-time stability study continues.
If you are interested in developing a bioresorbable surgical mesh and want a partner with experience, contact us for more information!
1 Lambert, B., et al. (2011). AAPS PharmSciTech, 12(4), pp. 1116-1126. doi: 10.1208/s12249-011-9644-8