Resorbable Ligation Device Elicits Successful Preliminary Results
Poly-Med, Inc. (PMI) is thrilled to share the success of the LigaTie®, a device designed and developed by Resorbable Devices AB in Uppsala, Sweden that utilizes one of Poly-Med’s Glycoprene® polymers. The LigaTie® device was developed by Dr. Odd Viking Höglund (http://bit.ly/innovator-LigaTie) and addresses challenges associated with ligation during surgical procedures. The device is utilized to restrict blood flow, prevent blood loss, and prevent air leaks, when used on lungs or airways. PMI supports Resorbable Devices AB in the material development of a novel, flexible, fast degrading, absorbable polymer that has been able to meet the demanding specifications of the LigaTie® device. The current product scope initially focuses on veterinary applications, and Resorbable Devices AB has seen great success with clinical results to date.
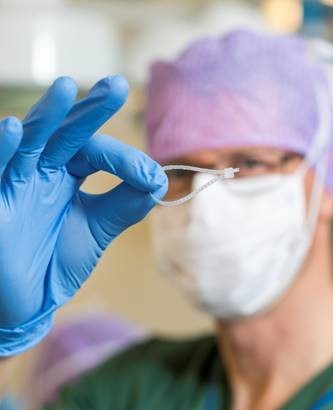
The LigaTie® has been successful in the following veterinary procedures: in vivo canine neutering, ligation of ovarian pedicles 1, 2 and spermatic cords,3 ex vivo cholecystectomies (removal of gallbladder) to seal the cystic duct,4 ex vivo sealing of lung tissue at lung biopsies,5 in vivo lung lobectomy in dogs with lung cancer,6 and a video-assisted thoracoscopic lung lobectomy (removal of lung lobe).7 The LigaTie® design is based on the concept of a cable tie, and the design allows for ligation of a single artery. The device is a flexible, unidirectional, self-locking, loop device produced from one of PMI’s Glycoprene® absorbable materials .
Permanent surgical sutures, and other non-absorbable devices (i.e., clips, cable ties, staples), may be used for ligation applications, but threaten to cause negative tissue responses, such as infection, inflammation, or chronic granulomas/scar tissue formation. On the other hand, the LigaTie® exhibits the following benefits due to the novel design and material choice: good tissue grip, easy and minimally invasive placement, which results in a reduction in surgery time (compared to suture ligation), standardized and secure locking mechanism, minimal inflammatory reactions, and acceptable responses for the mechanical performance-to-resorption profile. The preliminary results for the LigaTie® device are extremely promising and truly offer an innovative product for tissue ligation to prevent hemorrhage or leakage of air.
PMI is excited to support companies working to address challenges in the biomedical engineering and biotechnology fields. With PMI’s vertically integrated structure, they are capable of assisting clients take their ideas from exploration and investigation to final manufacturing and market, through in-house material development, analytical testing, product development, and project management. Connect with PMI today to hear more about our material offerings and design, development, and analytical capabilities! If you are interested in hearing more about how Poly- Med can help advance your idea or product, contact us today!
1 Höglund, O., et al. (2013). 27(8), pp.961-6. doi: 10.1177/0885328211431018.
2 Da Mota Costa, M., et al. (2016). BMC Res Notes, 9(245), pp. 1-6. doi: 10.1186/s13104-016-2042-2.
3 Höglund, O., et al. (2014). BMC Res Notes, 7(825), pp. 1-7. doi: 10.1186/1756-0500-7-825.
4 Tepper, S., et al. (2017). Can J Vet Res, 81(3), pp. 223-7. PMID: 28725113.
5 Nylund, A. et al. (Accepted). Vet Surg. Evaluation of a resorbable self-locking ligation device for performing peripheral lung biopsies in a caprine cadaveric model.
6 Ishigaki et al. (2017). Presentation at ACVS Surgery Summit. doi 10.1111/vsu.12710.
7 Guedes, R., et al. (2018). Surg Innov., 25(2), pp. 158-164. doi: 10.1177/1553350617751293. Link to video.