Bioresorbable Polymers for Implantable Medical Devices
Bioresorbable Polymers Overview:
Bioresorbable polymers (also referred to bioabsorbable polymers, resorbable polymers, absorbable polymers, and degradable polymers) are synthetic or naturally-derived materials exhibiting high-biocompatibility and degrade into smaller molecules that may be safely excreted from a patient. Bioresorbable polymers may degrade through mechanisms including but not limited to bulk hydrolysis, surface erosion, or enzymatic degradation depending on the material. Most implant-grade bioresorbable polymers polyester-based materials that are made of the following five (5) monomeric constituents: 1) glycolide, 2) L-lactide, 3), p-dioxanone, 4) ɛ-caprolactone, and 5) trimethylene carbonate. These five (5) monomeric constituents are then polymerized via a tin(II) catalyzed reaction to form an intended polymer.
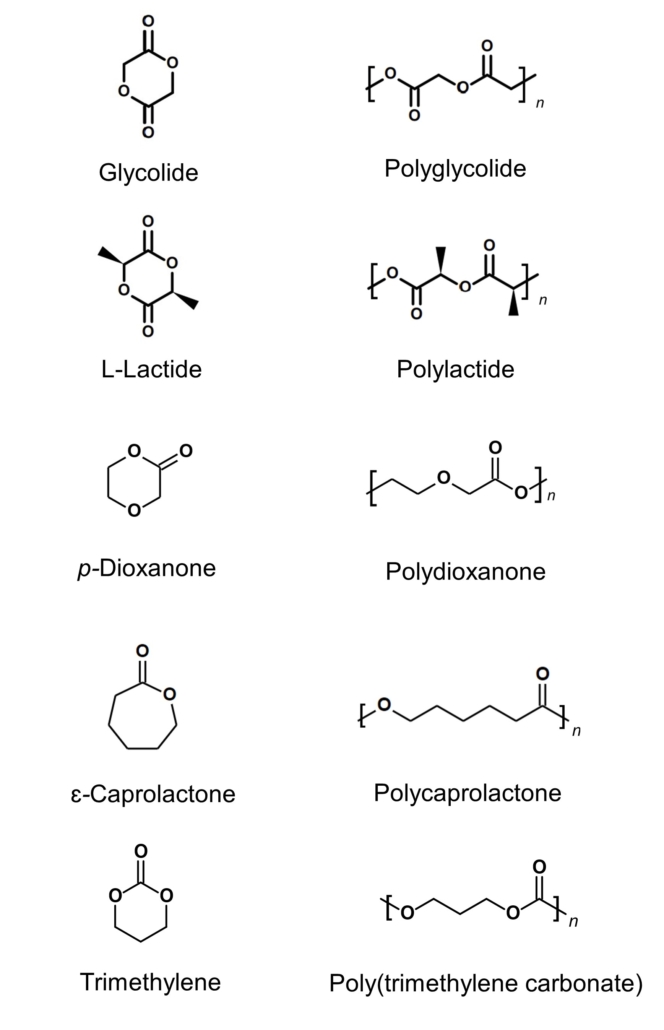
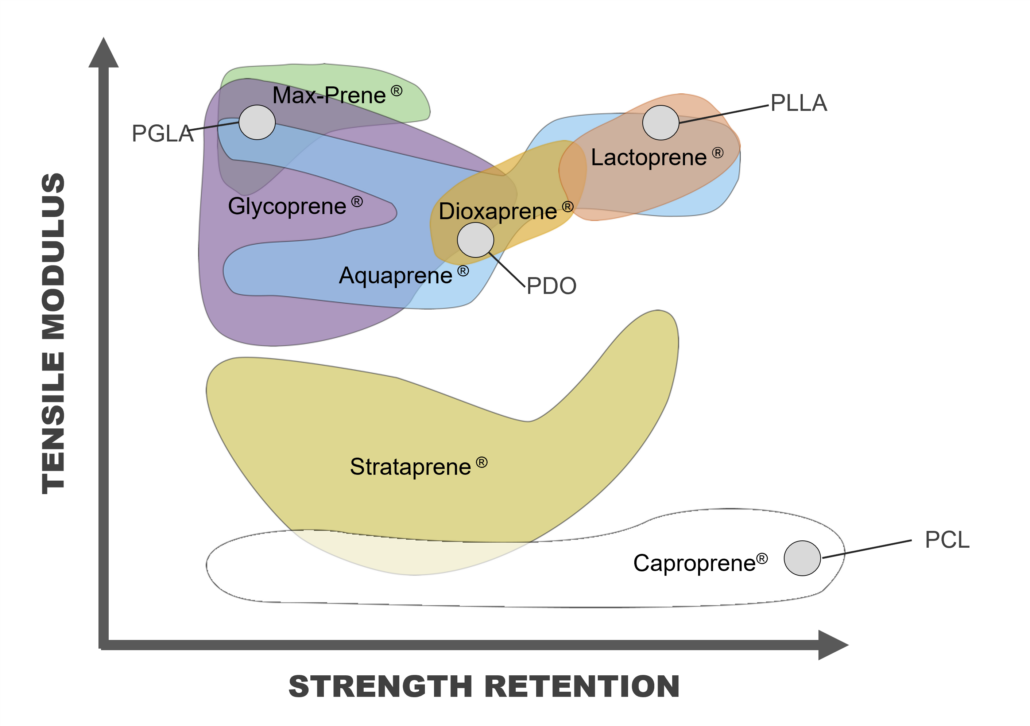
Typically, degradable medical devices are produced with off-the-shelf traditional homo-polymers or random co-polymers such polyglycolic acid (PGA), polylactic acid (PLA), polydioxanone (PDO), polycaprolactone (PCL), poly(lactic-co-glycolic acid) (PLGA), , or poly(L-lactide-co-ε-caprolactone) (PLCL). Although readily available from multiple bioresorbable polymer suppliers, often these materials lack ideal characteristics for implantable medical devices due to their mechanical properties or degradation profiles for intended applications (Figure 2).
Poly-Med’s Advanced Bioresorbable Polymers:
Poly-Med offers off-the-shelf high-performance bioresorbable polymers suitable for any intended absorbable medical device application. Typically, these materials are more flexible and exhibit decreased crystallinity compared to more commonly used homo- or co-polymers in use. The section below outlines the advantages to selecting one of Poly-Med’s high-performance bioresorbable polymers over homo-polymers or random co-polymers (Figure 3).
Glycoprene® Polymer Series as an alternative to PGA and PGLA polymers:
PGA and PGLA polymers may exhibit tensile strengths in the GPa range and degradation profiles of strength loss within one (1) to six (6) weeks and mass loss within two (2) to four (4) months. Poly-Med’s Glycoprene® bioresorbable polymer series are polyaxial, segmented co-polymer formulations with varying ratios of glycolide, ε-caprolactone, and trimethylene carbonate (TMC) monomers (Figure 3). With this polymer architecture, TMC acts as a central flexible region that can then be end-grafted with varying ratios of glycolide and ε-caprolactone, creating a triadic structure (Figure 4). Poly-Med’s stiffer Glycoprene® 935 has a composition of 93% glycolide, 5% ε-caprolactone, & 2% TMC and exhibits degradation profiles with strength loss ranging from one (1) to four (4) weeks and mass loss ranging from three (3) to six (6) months depending on processing, physiological environment, and anatomical location. Additionally, Poly-Med offers Glycoprene® 7424 as a formulation exhibiting increased flexibility with a composition of 74% glycolide, 24% ε-caprolactone, and 2% TMC. Similar to Glycoprene® 935, Glycoprene® 7424 exhibits a degradation profile with strength loss ranging from two (2) to four (4) weeks and mass loss ranging from three (3) to six (6) months. These materials may be processed into extruded monofilaments, multifilaments, tubes, films, warp and weft knit meshes, electrospun fabrics, FDM 3D printed filaments & parts, and injection molded constructs. When processed in a multifilament warp knit mesh construct, our Glycoprene® 935 material exhibits increased burst strength and elongation at break compared to Ethicon’s Vicryl® mesh made from a PGLA 90% glycolide / 10% L-lactide multifilament yarn.
Dioxaprene® Polymer Series as a medium-lasting material with
Polydioxanone was first introduced in the form of the PDS II monofilament suture product line by Ethicon in 1982. Polydioxanone is a linear homopolymer made from p-dioxanone and is exhibits advantageous biocompatibility properties due to decrease acid generation compared to glycolide- and lactiade-based materials. Poly-Med offers Dioxaprene® 100M polydioxanone material that exhibits strength loss in four (4) to six (6) weeks and mass loss in six (6) to nine months. Polydioxanone materials may be processed into extruded monofilaments & films, warp & weft knit meshes, electrospun fabrics, FDM 3D printed filaments & parts, and injection molded constructs.
Lactoprene® Polymer Series as an Alternative to PLLA, PLGA, and PLCL polymers:
PLLA and PLGA materials typically exhibit strengths in the GPa range, although with degradation profiles losing strength within six (6) to twelve (12) months and mass loss ranging eighteen (18) to thirty-six (36) months depending on processing, physiological environment, and anatomical location. 100% PLLA materials have shown problematic long-term biocompatibility when processed into certain formats, most notably with the recall of Ethicon’s Panacryl® suture for orthopedic applications due to burst release of lactic acid units from high-crystalline polymer segments.
Poly-Med’s Lactoprene® bioresorbable polymers are linear block co-polymer formulations with varying ratios of lactide, ε-caprolactone, and TMC (Figure 5). This polymer architecture creates a segmented degradation profile that does not exhibit burst release of lactic acid that has been observed with standard PLA polymers and therefore increases a devices biocompatibility potential. Similar to the triadic design implemented into the Glycoprene® and Strataprene® bioresorbable polymer series, the Lactoprene® bioresorbable polymer series also exhibits stiffer lactide-based segments and more flexible TMC-based segments. Poly-Med’s stiffest and longest lasting lactide-based material, Lactoprene® 8812, is constituted by 88% L-lactide & 12% TMC and exhibits strength loss ranging from six (6) to twelve (12) months and mass loss ranging from eighteen (18) to thirty-six (36) months. Poly-Med also offers a slightly more flexible formulation, Lactoprene® 8411, made of 84% L-lactide, 11% TMC, and 5% ε-caprolactone that exhibits a strength loss ranging from three (3) to nine (9) months and mass loss ranging from twelve (12) to thirty-six (36) months. Poly-Med also offers a unique, flexible lactide-based bioresorbable polymer formulation, Lactoprene® 7415, exhibiting a block co-polymer structure of 74% L-lactide, 11% ε-caprolactone, and 15% TMC. Additionally, Lactoprene® 7415 exhibits a similar degradation profile to Lactoprene® 8411 dependent on processing, physiological environment, and anatomical location. Overall, Poly-Med’s Lactoprene® bioresorbable polymer series may be used in a variety of manufacturing techniques that include but are not limited to extruded monofilaments, multifilaments, tubes, films, warp and weft knit meshes, electrospun fabrics, FDM 3D printed filaments & parts, and injection molded constructs.
Strataprene® Polymer Series as Unique Elastomeric Bioresorbable Polymer Materials:
Similar to Poly-Med’s Glycoprene® bioresorbable polymer series, Strataprene® polymer formulations exhibit a polyaxial block co-polymer architecture but are elastomeric materials. Our Strataprene® 5525 material exhibits extensibility of >500% when processed into a variety of formats. This bioresorbable polymer formulation is constituted by 55% glycolide, 25% TMC, and 20% ε-caprolactone. Strataprene® 5525 exhibits a strength loss ranging from one (1) to two (2) months and mass loss ranging from six (6) to nine (9) months depending on processing, physiological environment, and anatomical location. Strataprene® 5525 may be used for extruding films, electrospinning, FDM 3D printing filaments and parts, and injection molding.
Strataprene® 3534 exhibits similar mechanical properties to Strataprene® 5525, although much more extensible at >1,000%. This bioresorbable polymer formulation is constituted by 35% ε-caprolactone, 34% L-lactide, 17% glycolide and 14% TMC. Strataprene® 3534 exhibits a strength loss ranging from one (1) to three (3) months and mass loss ranging from twelve (12) to eighteen (18) months depending on processing, physiological environment, and anatomical location. Strataprene® 3534 may also be used for coating other materials, extruding films, electrospinning, FDM 3D printing filaments and parts, and injection molding.
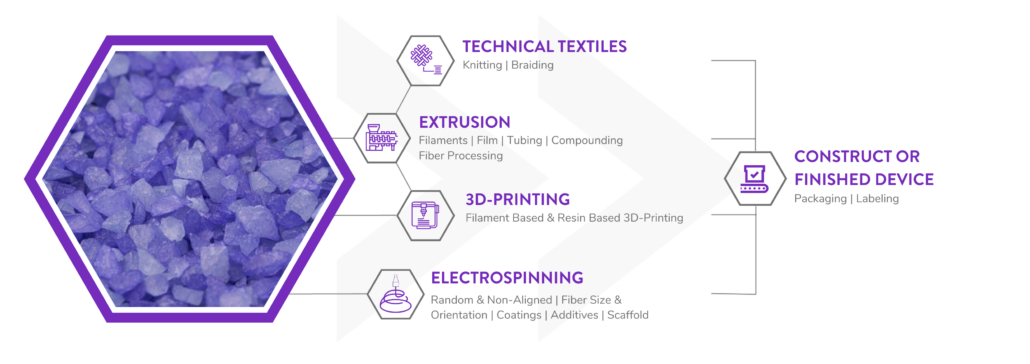
Poly-Med is a Vertically Integrated Bioresorbable Medical Device Manufacturing Partner:
Manufacturing bioabsorbable medical devices is hard. Controlling moisture levels and material degradation through the production cycle requires specialized equipment and process controls to ensure quality of finished devices. In contrast to other bioresorbable polymer manufacturers, Poly-Med produces polymer, is able to extrude this material to desired monofilament or multifilament formats, and is able process this material via warp knitting, weft knitting, or braiding processes to produce custom biomedical textiles. Beyond traditional textiles, we can process our bioresorbable polymers into non-woven formats via electrospinning via our state-of-the-art electrospinning facility. In addition to accessing Poly-Med’s more than thirty (30) years of experience manufacturing bioresorbable polymers, partnering with Poly-Med simplifies your bioresorbable medical device manufacturing supply chain. Contact us to today begin developing your custom bioresorbable medical device product line with a trusted partner for manufacturing advanced bioresorbable medical devices or purchase off-the-shelf high-performance bioresorbable polymers!