Biomedical Textiles as Implantable Medical Devices
Biomedical Textiles Overview:
Biomedical textiles are fiber-based medical devices that can be implanted in patients to restore tissue properties, provide mechanical support, as well as facilitate the healing process for a variety of indications. Textile-based implantable medical devices include but are not limited to sutures, meshes, vascular grafts, and heart valves. Additionally, biomedical textiles may be created from synthetic or natural sources and may be non-resorbable or resorbable based on the medical device’s intended use. Biomedical textiles may be constructed via one (1) of four (4) main processing methods: 1) knitting, 2) weaving, 3) braiding, and 4) non-woven methods (i.e. electrospinning, melt-blowing, air-laying, etc.). Each of these methods offer pros and cons for developing an implantable biomedical textile-based medical device and will be explored below.
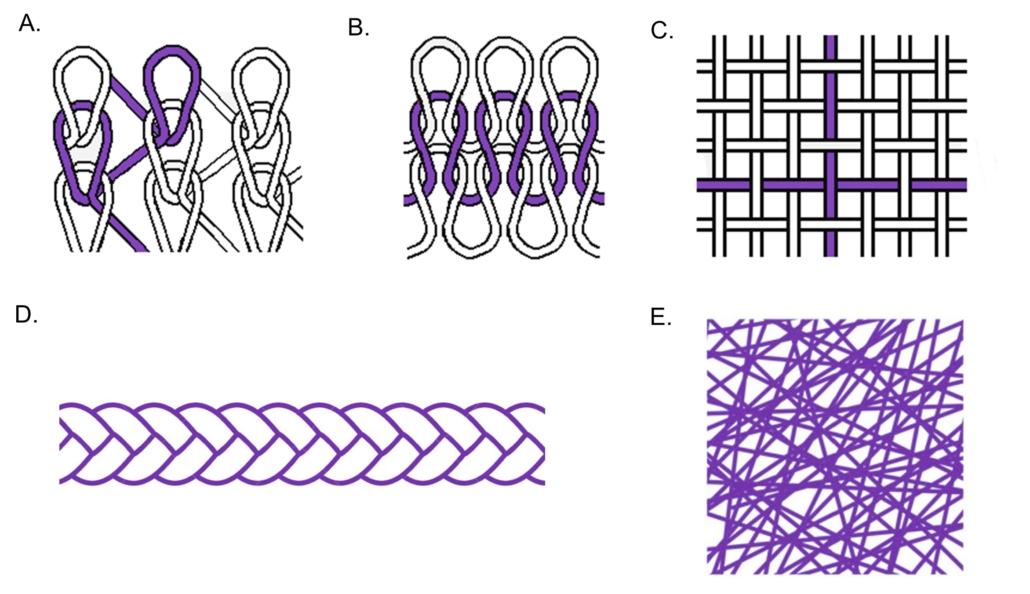
Knitted Biomedical Textile Constructs:
Knitted biomedical textiles are created by inter-looping yarns to form multi- dimensional structures ranging from planar fabric to custom 3D shapes.. Knitted materials may be created via warp or weft knitting processes. Warp knitted constructs have yarns spanning the length of the fabric, whereas weft knitted constructs have yarns spanning the width of the fabric (Figure 1A, 2). Typically, warp knitted materials may be created in flat formats, whereas weft knitted materials are created in tube-like structures. Due to differences in their yarn construction patterns, warp and weft knit materials exhibit differences in mechanical properties. Warp knitted materials exhibit increased tensile strength & modulus, and lower elongation values compared to weft knit materials of similar fiber diameters and areal densities (Figure 1B). Choice of warp or weft knit constructions largely depends on the intended use for the biomedical textile application. Knitted medical devices include but are not limited to hernia meshes, grafts, and envelopes for holding other biocompatible materials.

Figure 2: Examples of warp knitted absorbable mesh constructions.
Woven Biomedical Textile Constructs:
Woven biomedical textiles are created by interlacing of multiple sets of yarns at 90° angles using a loom (Figure 1C). Within this construct, the yarns running the length of the fabric are referred to as the warp direction, and the yarn filaments running the width of the fabric are referred to as weft direction. For 2D type structures, three (3) basic yarn patterns commonly woven are 1) plain, 2) twill, and 3) satin patterns. Plain weave patterns are constructed by passing each weft yarn over and under each warp yarn, alternating with each row. Twill weave patterns produce a fabric with a diagonal wale, created by an offset of the warp yarns. For the satin weave pattern, the weft yarns are predominantly on the front of the fabric and the interlacing weft yarns are spaced as widely as possible. Because the interlacing yarns are scattered and not a set distance, a line does not form as is the case of the twill pattern. Similar to, knitted constructs, patterns of woven materials influence the mechanical properties of the material, making certain constructions more or less appropriate given the intended use of the implantable medical device. Woven biomedical textiles are currently in use for vascular grafts as they exhibit high tensile strength in both the machine and counter-machine direction, high friction resistance, and low elongation.
Braided Biomedical Textile Constructs:
Braided biomedical textiles are created by interlacing three (3) or more yarns in diagonally overlapping patterns to form strand-like constructions (Figure 1D, 3). These constructions include but are not limited to solid braids, hollow core braids, multi-layer braids, flat braids, bifurcated braids, and discs. Braided constructs may be customized to have a desired number yarn ends to ensure certain design features and mechanical properties are met. Common braided biomedical textiles include but are not limited to sutures, stents, shunts, and expandable medical devices.

Figure 3: Example of absorbable multifilament braid construct.
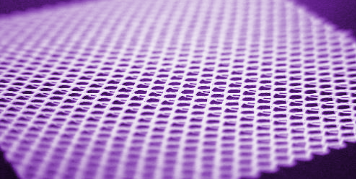
Non-Woven Biomedical Textile Constructs:
Non-woven biomedical textiles exhibit web-like structures of entangled fibers formed via mechanical, thermal, chemical, or electrical processing methods (Figure 1E, 4). Non-woven materials may exhibit fiber sizes on the order of extracellular matrices to act as tissue scaffolds to aid in the healing process. Currently marketed implantable biomedical textiles with non-woven nanofibers include melt-blown materials for hernia repair exhibiting diameters from –20 – 50 microns and electrospun materials used for wound matrices exhibiting fiber diameters form 0.05 – 1 microns.
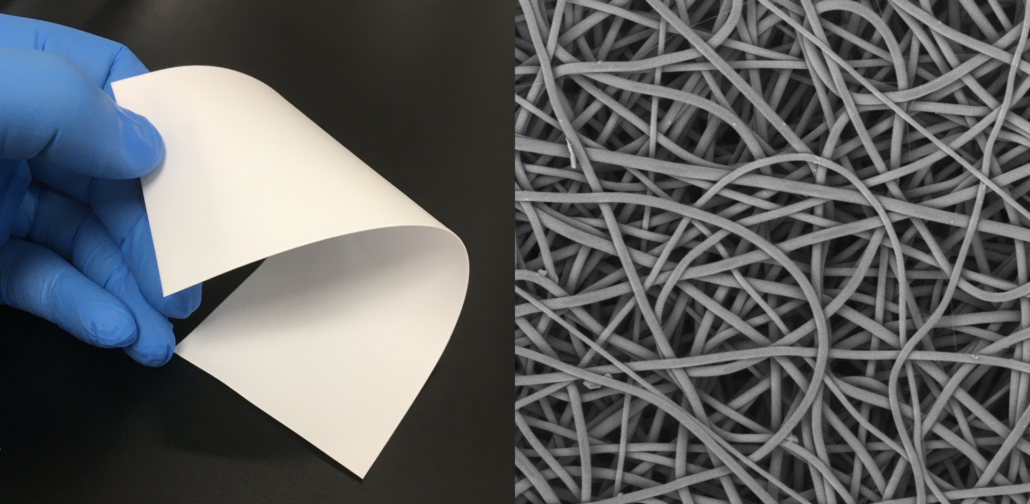
Figure 4: Example of absorbable electrospun non-woven fabric.
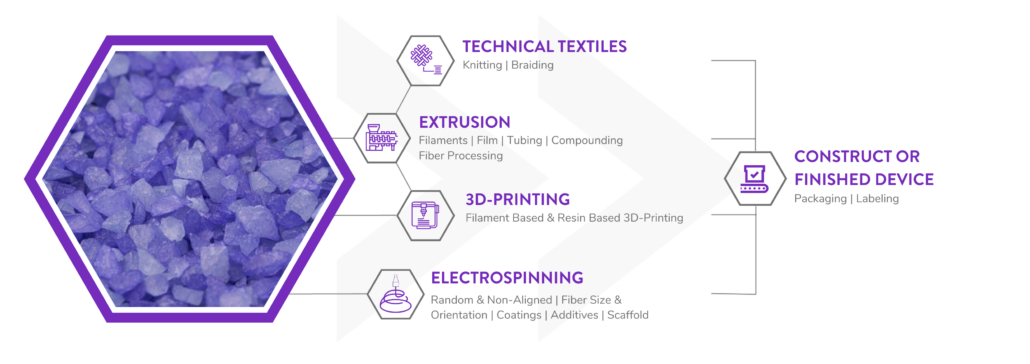
Poly-Med is a Vertically Integrated Bioresorbable Medical Device Manufacturing Partner:
Manufacturing bioabsorbable medical devices is hard. Controlling moisture levels and material degradation through the production cycle requires specialized equipment and process controls to ensure quality of finished devices. In contrast to other bioresorbable polymer manufacturers, Poly-Med produces polymer, is able to extrude this material to desired monofilament or multifilament formats, and is able process this material via warp knitting, weft knitting, or braiding processes to produce custom biomedical textiles. Beyond traditional textiles, we can process our bioresorbable polymers into non-woven formats via electrospinning via our state-of-the-art electrospinning facility. In addition to accessing Poly-Med’s more than thirty (30) years of experience manufacturing bioresorbable polymers, partnering with Poly-Med simplifies your bioresorbable medical device manufacturing supply chain. Contact us to today begin developing your custom bioresorbable medical device product line with a trusted partner for manufacturing advanced bioresorbable medical devices or purchase off-the-shelf high-performance bioresorbable polymers.