Advanced Absorbable Implants.
BIORESORBABLE SOLUTIONS DELIVERED
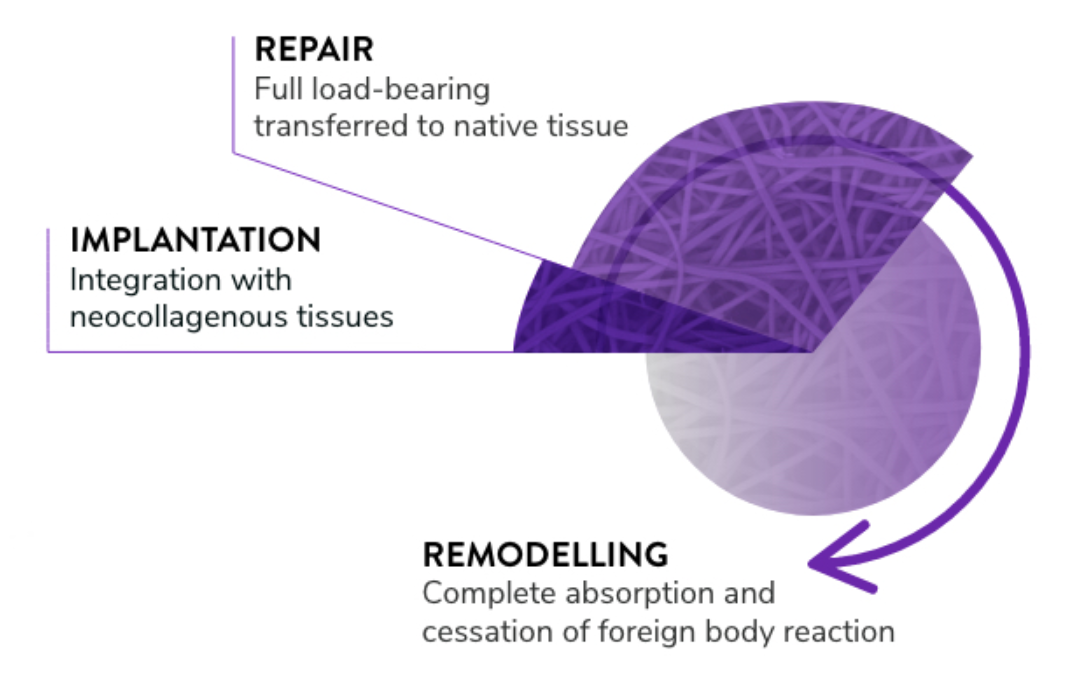
Materials, devices, and delivery materials that work with the body for better, faster outcomes
Advanced Absorbable Implants
BIORESORBABLE SOLUTIONS DELIVERED
Materials, devices, and delivery materials that work with the body for better, faster outcomes.